Term Used to Describe Force Holding Molecules Together
Select all the statements that. Induced dipole forces A great example of hydrogen bonding holding molecules together to.

Interintramolcforces Gif 500 330 Teaching Chemistry High School Chemistry Chemistry
Intermolecular and intramolecular forces are the two types of forces that hold individual molecules and atoms together.
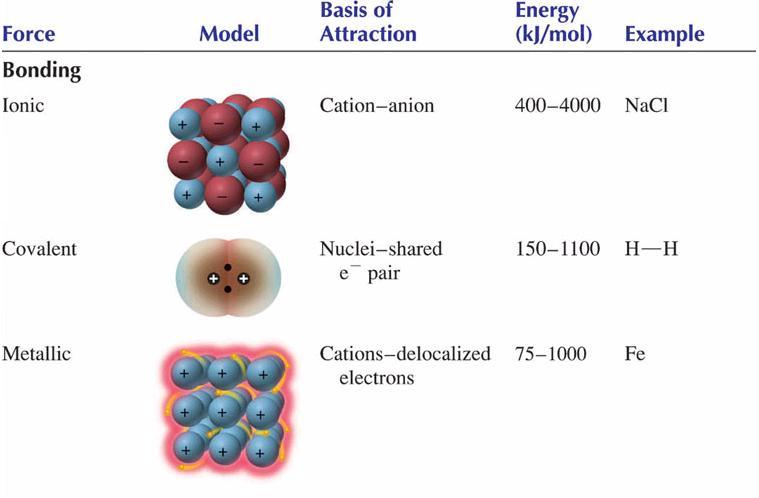
. A chemical bond is a force that holds two atoms together in a molecule. These forces mediate the interactions between individual molecules of a substance. What term is used to describe all of the chemical reactions that occur in body cells.
The sum of the attractive or repulsive forces between molecules or between parts of the same molecule other than those due to covalent bonds or the electrostatic interaction of ions with one another with neutral molecules or with charged molecules. A chemical bond formed by the sharing of one or more pairs of electrons between the outer shells of two atoms is called an _____ bond. In the following description the term particle will be used to refer to an atom molecule or ion.
London Dispersion and hydrogen bond charges are the intermolecular forces holding the molecules together 8. Intermolecular forces are responsible for most of the physical and chemical properties of matter. J The overall change in the molecule caused by the introduction of a carbon-carbon double bond is the bent shape it gives which results in atoms not fitting as close together when they were just single bonds originally.
Van der Waals Forces. If youre seeing this message it means were having trouble loading external resources on our website. And number two we are looking for inter molecular forces.
Describe the intermolecular forces that hold bromine molecules together in the liquid state between the temperatures of 72C and 588C and in the solid state below 72C. A force holding two atoms together is an chemical bond. The forces holding atoms together are generally electrostatic forces gravitational forceslittle significance vander waal forces of attraction.
Chemical bond is the force that hold two atoms. As was the case for gaseous substances the kinetic molecular theory may be used to explain the behavior of solids and liquids. The forces that hold the _____ together in compounds and molecular elements are called chemical _____.
This is the term to describe the attraction between one nonpolar molecule and another nonpolar molecule. The water molecule has two distinct ends each with. Describe chemical bonding.
Covalent bonds are very common in non metallic compounds and elements. This is the force inside a molecule of bromine hold the molecule together. Both types of forces determine the chemical and physical characteristics of substances.
Note that we will use the popular phrase intermolecular attraction to refer to attractive forces between the particles of a substance regardless of whether these. So I am forces are forces between molecules that hold molecules together not the forces that hold atoms together or. Chemical bonding is the general term used to describe the forces that hold atoms together in molecules and ions.
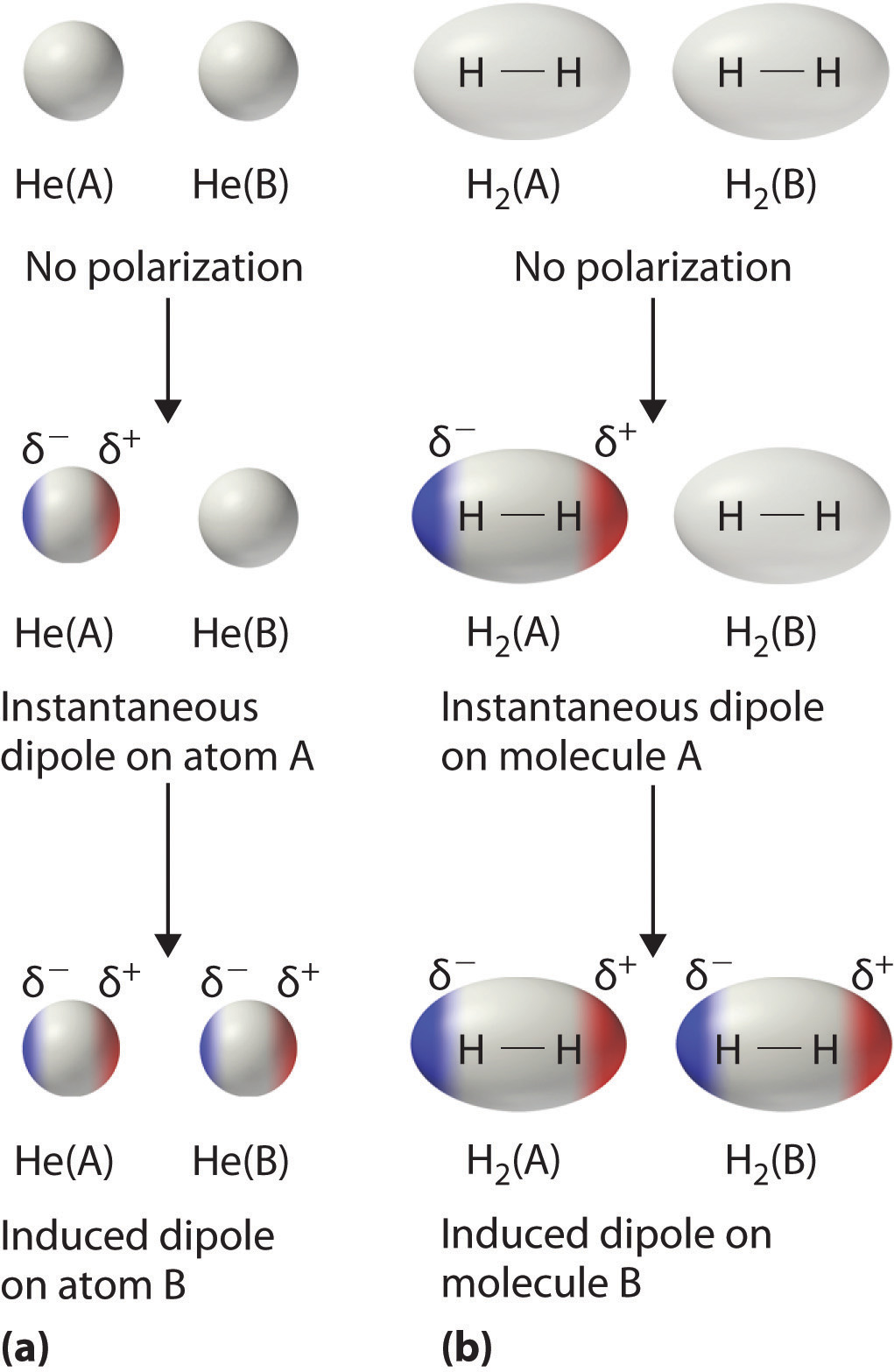
Dispersion forces are however the only intermolecular force experienced by. Intermolecular forces are the forces of attraction that exist between covalent compounds holding them together. Chemical bonds hold molecules together and create temporary connections that are essential to life.
Dispersion forces are experienced by ALL particles atoms ions and molecules. Salt linkages ionic bonds result from interactions between positively and negatively charged groups on the side chains of the basic and acidic amino acids. Introduction to Chemistry 4th Edition Edit edition Solutions for Chapter 10 Problem 140AQ.
So what that results in is covalin bonding. The SNF is responsible for holding the protons in a nucleus together against the force of their electrostatic repulsion due to having like charges. But also very common in simple covalent compounds is the concept of the intermolecular force.
This is the force between two molecules of bromine holds the molecules together Dipole dipole. A force holding two atoms together is an A. What term is used to describe splitting a large atomic nucleus into two smaller ones.
Types of chemical bonds including covalent ionic and hydrogen bonds and London dispersion forces. These forces control the movement of molecules and atoms. Its a correct answer.
Two idealized types of bonding are ionic bonding in which positively and negatively charged ions are held together by electrostatic forces and covalent bonding in which electron pairs are. Match the term concerning hydrocarbon in the left column with the correct description in the right column. Such molecules are said to be A.
Intermolecular forces often abbreviated to IMF are the attractive and repulsive forces that arise between the molecules of a substance. There are three different types of different strengths. The main difference between intermolecular and intramolecular forces is that intermolecular.
Van der Waals forces. I can see how repulsive forces can occur when molecules are held close together due to interaction of negatively charged electrons like charges repel.
12 6 Types Of Intermolecular Forces Dispersion Dipole Dipole Hydrogen Bonding And Ion Dipole Chemistry Libretexts

Intermolecular Force Easy Science Intermolecular Force Force Definition Easy Science
Comments
Post a Comment